Medicine
Health services research in the United States has historically been the "poor cousin" of biomedical research in federal funding and support. The annual budget for the National Institutes of Health, for example, is typically around 100 times that of the Agency for Healthcare Research and Quality (AHRQ). In last month's Georgetown University Health Policy seminar, we discussed the financial and political challenges that AHRQ and its predecessor, the Agency for Health Care Policy and Research (AHCPR) have faced while trying to improve outcomes and effectiveness of medical care since the latter's founding during the first Bush Administration.

Citing John Wennberg's pioneering geographic analyses of medical practice variations and potentially inappropriate use of health services across the U.S., AHCPR's supporters wanted the new agency to produce practice guidelines to promote evidence-based care. However, when one of those guidelines suggested that spinal fusion surgery was unnecessary for most patients with acute low back pain, AHCPR found its budget under attack. It didn't help that the agency was also identified with the failed Clinton health reform plan and had few defenders left in a Republican Congress after the 1994 elections. Although the agency survived, this experience eventually drove it out of the guideline-producing business for good. When AHRQ was reauthorized in 1999, the word "policy" was removed from its name.
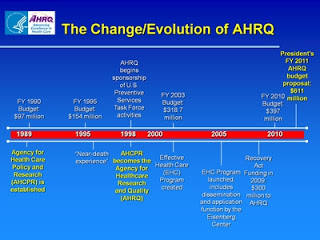
It's understandable that this episode made future AHRQ leaders reluctant to wade into explosive scientific controversies, especially regarding sacred cows of medicine such as mammography and prostate cancer screening. When the AHRQ-supported U.S. Preventive Services Task Force chose to do so, the political fallout again put the agency in an uncomfortable position. Distancing itself from the USPSTF's recommendation against routine mammography in women younger than 50 and repeatedly delaying the release of another that advised clinicians to stop prostate-specific antigen testing altogether, AHRQ still found itself under heavy fire from health reform opponents on Capitol Hill. In July 2012, it was deja-vu all over again as AHRQ's budget was singled out for elimination by an appropriations subcommittee in the House of Representatives. Supporters of health services research in the U.S. lined up to defend the agency. The bill was not taken up by the Senate, and of this writing, AHRQ appears to have survived another "near death experience."
**
The above post first appeared on The Health Policy Exchange. Note: I was employed as a medical officer at AHRQ from October 2006 through December 2010.
- Clinical Guidelines And Technology Assessment
“Clinical Guidelines and Technology Assessment”? Well, there’s a yawner! Maybe you'll be one of the few who won’t say “I think I’ll skip this one”, but if you are not, I’d have to be empathic. I’m thinking about the time I picked...
- The End Of The Line On Psa Screening
In the summer of 2007, then-U.S. Preventive Services Task Force member Russ Harris, MD, MPH approached me about taking on what he suggested would be a fairly quick and straightfoward project: summarizing the small amount of medical literature on...
- The Meeting That Wasn't, Revisited
A New York Times Magazine story published on the newspaper's website this morning details the complicated history of screening for prostate cancer in the U.S. and revisits the related story of the U.S. Preventive Services Task Force meeting that was...
- How Politically Unpopular Research Helps Us Make Better Medical Decisions
When a new drug goes on the market for, say, diabetes, doctors are typically bombarded by advertising messages that promote it. Patients may see television commercials touting the new drug’s advantages over older ones and advising them to "talk to your...
- Psa Testing: Will Science Finally Trump Politics?
Maybe the third time will finally be the charm.In early November 2009, the U.S. Preventive Services Task Force voted unanimously to update its 15-month old recommendations on screening for prostate cancer in men younger than 75, changing its previous...
Medicine
Politics and practice guidelines: a volatile mix
Health services research in the United States has historically been the "poor cousin" of biomedical research in federal funding and support. The annual budget for the National Institutes of Health, for example, is typically around 100 times that of the Agency for Healthcare Research and Quality (AHRQ). In last month's Georgetown University Health Policy seminar, we discussed the financial and political challenges that AHRQ and its predecessor, the Agency for Health Care Policy and Research (AHCPR) have faced while trying to improve outcomes and effectiveness of medical care since the latter's founding during the first Bush Administration.

Citing John Wennberg's pioneering geographic analyses of medical practice variations and potentially inappropriate use of health services across the U.S., AHCPR's supporters wanted the new agency to produce practice guidelines to promote evidence-based care. However, when one of those guidelines suggested that spinal fusion surgery was unnecessary for most patients with acute low back pain, AHCPR found its budget under attack. It didn't help that the agency was also identified with the failed Clinton health reform plan and had few defenders left in a Republican Congress after the 1994 elections. Although the agency survived, this experience eventually drove it out of the guideline-producing business for good. When AHRQ was reauthorized in 1999, the word "policy" was removed from its name.
From 2010 AHRQ Annual Conference presentation by Dr. Francis Chesley, Jr.
**
The above post first appeared on The Health Policy Exchange. Note: I was employed as a medical officer at AHRQ from October 2006 through December 2010.
- Clinical Guidelines And Technology Assessment
“Clinical Guidelines and Technology Assessment”? Well, there’s a yawner! Maybe you'll be one of the few who won’t say “I think I’ll skip this one”, but if you are not, I’d have to be empathic. I’m thinking about the time I picked...
- The End Of The Line On Psa Screening
In the summer of 2007, then-U.S. Preventive Services Task Force member Russ Harris, MD, MPH approached me about taking on what he suggested would be a fairly quick and straightfoward project: summarizing the small amount of medical literature on...
- The Meeting That Wasn't, Revisited
A New York Times Magazine story published on the newspaper's website this morning details the complicated history of screening for prostate cancer in the U.S. and revisits the related story of the U.S. Preventive Services Task Force meeting that was...
- How Politically Unpopular Research Helps Us Make Better Medical Decisions
When a new drug goes on the market for, say, diabetes, doctors are typically bombarded by advertising messages that promote it. Patients may see television commercials touting the new drug’s advantages over older ones and advising them to "talk to your...
- Psa Testing: Will Science Finally Trump Politics?
Maybe the third time will finally be the charm.In early November 2009, the U.S. Preventive Services Task Force voted unanimously to update its 15-month old recommendations on screening for prostate cancer in men younger than 75, changing its previous...
